Cannabis and Arthritis
More than 31 million Americans suffer from arthritis. There are two main types of arthritis: rheumatoid arthritis and osteoarthritis. Both affect the joints, causing pain and swelling, and limiting movement.
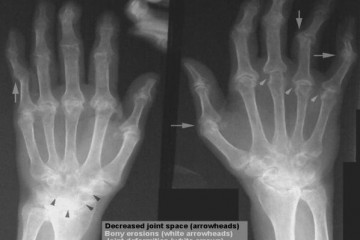
Rheumatoid arthritis (RA) is caused by a malfunction of the immune system. Instead of fighting off intruders such as bacteria or viruses, the body attacks the synovial membranes, which facilitate the movement of joints, eventually destroying cartilage and eroding bones. Rheumatoid arthritis is most common among the aged, whose immune systems are no longer as robust or efficient as they were when younger.
Osteoarthritis (OA), or arthritis of the bones, is also found primarily among the elderly, where cartilage has been worn away through many years of use. Arthritis may also manifest as chronic inflammation of the joints as the result of injuries. OA is the most common form of arthritis, affecting more than 10 million people worldwide. Currently, no drugs are available to treat or modify this disease, and treatment is primarily focused around the use of pain killers, which often have limited benefits and hazardous side effects.
An important aspect of arthritis pathology relates to maintaining healthy bone. As people age, bones undergo extensive remodeling, which can lead to destruction or functional degradation of synovial joints. Drugs which can not only modulate pain from arthritis but also protect bones are of great importance. Cannabis and cannabinoids represent a promising treatment which can reduce arthritic pain and inflammation and positively modulate bone growth and maintenance. It has already been demonstrated that cannabinoids can effectively treat some types of arthritic pain, but recent evidence suggests that the cannabinoids are also important for bone growth and maintenance throughout life.[12-17]
The importance of cannabinoids in bone health has been established in transgenic mice that are missing either the CB1 or CB2 receptor. These mice develop osteoporosis much more quickly than normal or wild mice. Research has recently shown that mice missing both cannabinoid receptors have extremely weak bones, a condition that underlies osteoporosis and osteoarthritis pathology.[18-20]
Based on genetic screening techniques, a correlation between cannabinoids and bone is emerging in humans as well. Three studies in three distinct ethnic groups have demonstrated that mutations in the type 2 cannabinoid receptor correlate to bone diseases. One study even showed that hand bone strength weakness is very well correlated with dysfunctional/mutant CB2 receptors.
Arthritis of any type can be an extremely painful and debilitating condition that presents challenges for pain management. The use of cannabis as a treatment for musclo-skeletal pain in western medicine dates to the 1700s.[21-22] Evidence from recent research suggests that cannabis-based therapies are effective in the treatment of arthritis and the other rheumatic and degenerative hip, joint and connective tissue disorders. Since these are frequently extremely painful conditions, the well-documented analgesic properties of cannabis make it useful in treating the pain associated with arthritis, both on its own and as an adjunct therapy that substantially enhances the efficacy of opioid painkillers.
Cannabis has also been shown to have powerful immune-modulation and anti-inflammatory properties,[23-26] suggesting that it could play a role not just in symptom management but treatment of arthritis. In fact, one of the earliest records of medical use of cannabis, a Chinese text dating from ca. 2000 BC, notes that cannabis “undoes rheumatism,” suggesting its anti-inflammatory and immune modulating effects were known even then.[27]
Modern research on cannabidiol (CBD), one of the non-psychoactive cannabinoid components of cannabis, has found that it suppresses the immune response in mice and rats that is responsible for a disease resembling arthritis, protecting them from severe damage to their joints and markedly improving their condition.[28-29]
Human studies have repeatedly shown cannabis to be an effective treatment for rheumatoid arthritis, and it is one of the enumerated conditions for which many states allow legal medical use. Cannabis has a demonstrated ability to improve mobility and reduce morning stiffness and inflammation. Research has also shown that patients are able to reduce their usage of potentially harmful Non-Steroidal Anti-Inflammatory drugs (NSAIDs) when using cannabis as an adjunct therapy.[30-31]
Medical researchers at Hebrew University in Jerusalem found that when cannabidiol is metabolized, one result is the creation of a compound with potent anti-inflammatory action comparable to the drug indomethacin, but without the considerable gastrointestinal side effects associated with that drug.[32]
In addition, when the body metabolizes tetrahydrocannabinol (THC), one of the primary cannabinoid components of cannabis, it produces a number of related chemicals. At least one of these metabolites has anti-inflammatory and pain-relieving effects. By modifying this metabolite, researchers have produced a synthetic carboxylic acid known as CT-3 (also called dimethylheptyl-THC-11 oic acid or DMH-11C ), which is more powerful than the natural metabolite itself, and thus can be given in smaller doses. Animal tests found CT-3 effective against both chronic and acute inflammation, and it also prevented destruction of joint tissue from chronic inflammation.
The remarkable 5,000-year safety record of cannabis – there has never been a recorded death from an overdose – and the fact that a metabolite with the desired anti-inflammatory effect is produced in the body when cannabis is used, indicates that the development of targeted, safe, and effective anti-inflammatory drugs in this class are possible.[33] CT3 has also demonstrated considerable analgesic effects in animals. In some cases, the dose-dependent effect of THC was equivalent to morphine, but with a much greater duration of action and far less toxicity.[34-35]
In contrast to the NSAIDs commonly prescribed arthritis sufferers, CT3 did not cause ulcers at therapeutically effective doses. Moreover, it does not depress respiration, produce dependence, induce body weight loss, or cause mutations, as many commonly prescribed drugs do. Studies on its mechanism of action are currently underway, with cytokine synthesis one of the pathways being studied.[36}
Cannabis may also help combat rheumatoid arthritis through its well-recognized immune-modulation properties.[37] Rheumatoid arthritis is characterized by ysregulation of the immune system in response to an initial infection or trauma. Over-activity of the immune system’s B-cells causes antibodies to attack and destroy the synovial tissues located in the joint.
The immuno-modulatory properties of a group of fats found in cannabis, known as sterols and sterolins, have been used as natural alternatives to conventional rheumatoid arthritis treatments that employ highly toxic drugs to either suppress the entire immune response of the body or to palliate pain and the inflammatory process without correcting the underlying immune dysfunction.
Cytokines play a role in either fuelling or suppressing the inflammation that causes damage in rheumatoid arthritis and some other diseases. The release of selected cytokines is impaired by cannabis, but the findings differ by cell type, experimental conditions, and especially the concentration of the cannabinoids examined.[38-41] A sterol/sterolin combination has been experimentally demonstrated to reduce the secretion of the pro-inflammatory cytokines controlled by the TH2 helper cells and to increase the number of TH helper cells that regulate the secretion of antibodies from the B cells. This selective activation and inhibition of the immune system results is an effective control of the dysfunctional auto-immune response.
Similarly, ajulemic acid (another non-psychoactive cannabinoid) has been found to reduce joint tissue damage in rats with adjuvant arthritis.[42] Tests on human tissue done in vitro showed a 50% suppression of one of the body’s chemicals (interleukin-1beta) central to the progression of inflammation and joint tissue injury in patients with rheumatoid arthritis.[43]
Conventional Arthritis Medications
Over 100 medications are listed by the Arthritis Foundation website for use with arthritis or other related conditions, such as fibromyalgia, psoriasis, osteoporosis and gout. These medicines include aspirin, ibuprofen and other oral and topical analgesics that dull pain. The most commonly used analgesic, acetaminophen (aspirin-free Anacin, Excedrin, Panadol, Tylenol) is usually not associated with side effects, though long-term use of acetaminophen is thought to be one of the common causes of end-stage renal disease.. To effectively control arthritis, aspirin must be taken in large, continuous doses (1000-5400 mg daily), which can cause stomach pain or damage; it is believed to cause more than 1,000 deaths annually in the United States. For that reason, some doctors prescribe one of several chemical variations referred to as nonacetylated salicylates, such as CMT, Tricosal, and Trilisate, which can cause deafness or ringing in the ears in large doses.
Much stronger analgesics are also prescribed for arthritis, sometimes along with acetaminophen. These are: codeine (Dolacet, Hydrocet, Lorcet, Lortab); morphine (Avinza, Oramorph); oxycodone (Vicodin, Oxycontin, Roxicodone); propoxyphene (Percocet, Darvon, Darvocet) and tramadol (Ultram, Ultracet). These medicines can cause psychological and physical dependence, as well as constipation, dizziness, lightheadedness, mood changes, nausea, sedation, shortness of breath and vomiting. Taking high doses or mixing with alcohol can slow down breathing, a potentially fatal condition.
Analgesics don’t treat the inflammation that can cause severe arthritis pain. For inflammation, steroids, NSAIDs and newer COX-2 inhibitors are prescribed. Corticosteroids (Cortisone), prednisone and related medications can cause bruising, cataracts, elevated blood sugar, hypertension, increased appetite, indigestion, insomnia, mood swings, muscle weakness, nervousness or restlessness, osteoporosis, susceptibility to infection and thin skin.
Twenty NSAIDs are available with a doctor’s prescription, with three of those also available over the counter. They are diclofenac (Arthrotec, Cataflam, Voltaren); diflunisal (Dolobid); etodolac (Lodine); fenoprofen calcium (Nalfon); flurbiprofen (Ansaid); ibuprofen (Advil, Motrin IB, Nuprin); indomethacin (Indocin); ketoprofen (Orudis); meclofenamate sodium (Meclomen); mefenamic acid (Ponstel); meloxicam (Mobic); nabumetone (Relafen); naproxen (Naprosyn, Naprelan); naproxen sodium (Anaprox, Aleve);oxaprozin (Daypro); piroxicam (Feldene); sulindac (Clinoril); and olmetin sodium (Tolectin).
Side effects of NSAIDs include abdominal or stomach cramps, edema (swelling of the feet), pain or discomfort, diarrhea, dizziness, drowsiness or lightheadedness, headache, heartburn or indigestion, nausea or vomiting, gastric ulcers, stomach irritation, bleeding, fluid retention, and decreased kidney function. This is because NSAIDs act on arthritis by inhibiting prostaglandins, which protect the stomach lining, promote clotting of the blood, regulate salt and fluid balance, and maintain blood flow to the kidneys. The gastrointestinal complications of NSAIDS are the most commonly reported serious adverse drug reaction, though NSAIDs are reported to cause more than 10,000 deaths and 100,000 hospitalizations annually.
The newer group of arthritis drugs is known as cyclo-oxygenase-2 inhibitors (COX-2), which include Celebrex, Bextra and Vioxx. These medications have the same side effects as NSAIDS, except they are less likely to cause bleeding stomach ulcers and increase susceptibility to bruising or bleeding.
Non-selective NSAIDS have been associated with an increased risk of congestive heart failure. Less is known or has been concluded about the cardiovascular effects f COX-2 inhibitors, though a retrospective analysis of the risk of hospital admission for heart failure done by the Institute for Clinical Evaluative Sciences in Toronto, Canada suggests some may have serious side effects. The study of 130,000 older patients found that those using Vioxx had an 80% increased risk of hospital admission for congestive heart failure. Those using non-selective NSAIDS had a 40% increased risk, and those using Celebrex had the same rate of heart failure as people who had never used NSAIDS.
Antipyretic and anti-inflammatory effects of NSAIDs can mask the signs and symptoms of infection. Their use can interfere with the pharmacologic control of hypertension and cardiac failure in patients who take beta-adrenergic antagonists, angiotensin-converting enzyme inhibitors, or diuretics. Long-term use may damage chondrocyte (cartilage) function.
About 60% of patients will respond to any single NSAID. Approximately 10% of rheumatoid arthritis patients will not respond to any NSAID. Biologic response modifiers such as adalimumab (Humira); etanercept (Enbrel); infliximab (Remicade), and anakinra (Kineret) are prescribed to either inhibit or the supplement the immune system components called cytokines. Rare reports of lupus (with such symptoms as rash, fever and pleurisy) have been linked to treatment with adalimumab, etanercept and infliximab. Lupus symptoms resolve when the medication is stopped. Multiple sclerosis has rarely developed in patients receiving biologic response modifiers. Seizures have been reported with etanercept.
Cannabis: By comparison, the side effects associated with cannabis are typically mild and are classified as “low risk.” Euphoric mood changes are among the most frequent side effects. Cannabinoids can exacerbate schizophrenic psychosis in predisposed persons. Cannabinoids impede cognitive and psychomotor performance, resulting in temporary impairment. Chronic use can lead to the development of tolerance. Tachycardia and hypotension are frequently documented as adverse events in the cardiovascular system. A few cases of myocardial ischemia have been reported in young and previously healthy patients. Inhaling the smoke of cannabis cigarettes induces side effects on the respiratory system. Cannabinoids are contraindicated for patients with a history of cardiac ischemia. In summary, a low risk profile is evident from the literature available. Serious complications are very rare and are not usually reported during the use of cannabinoids for medical indications.
Is cannabis safe to recommend?
“The smoking of cannabis, even long term, is not harmful to health….” So began a 1995 editorial statement of Great Britain’s leading medical journal, The Lancet. The long history of human use of cannabis also attests to its safety – nearly 5,000 years of documented use without a single death. In the same year as the Lancet editorial, Dr. Lester Grinspoon, a professor emeritus at Harvard Medical School who has published many influential books and articles on medical use of cannabis, had this to say in an article in the Journal of the American Medical Association (1995):
“One of marihuana’s greatest advantages as a medicine is its remarkable safety. It has little effect on major physiological functions. There is no known case of a lethal overdose; on the basis of animal models, the ratio of lethal to effective dose is estimated as 40,000 to 1. By comparison, the ratio is between 3 and 50 to 1 for secobarbital and between 4 and 10 to 1 for ethanol. Marihuana is also far less addictive and far less subject to abuse than many drugs now used as muscle relaxants, hypnotics, and analgesics. The chief legitimate concern is the effect of smoking on the lungs. Cannabis smoke carries even more tars and other particulate matter than tobacco smoke. But the amount smoked is much less, especially in medical use, and once marihuana is an openly recognized medicine, solutions may be found; ultimately a technology for the inhalation of cannabinoid vapors could be developed.”
The technology Dr. Grinspoon imagined in 1995 now exists in the form of “vaporizers,” (which are widely available through stores and by mail-order) and recent research attests to their efficacy and safety.[44] Additionally, pharmaceutical companies have developed sublingual sprays and tablet forms of the drug. Patients and doctors have found other ways to avoid the potential problems associated with smoking, though long-term studies of even the heaviest users in Jamaica, Turkey and the U.S. have not found increased incidence of lung disease or other respiratory problems. A decade-long study of 65,000 Kaiser-Permanente patients comparing cancer rates among non-smokers, tobacco smokers, and cannabis smokers found that those who used only cannabis had a slightly lower risk of lung and other cancers as compared to non-smokers.[45] Similarly, a study comparing 1,200 patients with lung, head and neck cancers to a matched group with no cancer found that even those cannabis smokers who had consumed in excess of 20,000 joints had no increased risk of cancer.[46]
As Dr. Grinspoon notes, “the greatest danger in medical use of marihuana is its illegality, which imposes much anxiety and expense on suffering people, forces them to bargain with illicit drug dealers, and exposes them to the threat of criminal prosecution.” This was the conclusion reached by the House of Lords, which recommended rescheduling and decriminalization.
Cannabis or Marinol?
Those committed to the prohibition on cannabis frequently cite Marinol, a Schedule III drug, as the legal means to obtain the benefits of cannabis. However, Marinol, which is a synthetic form of THC, does not deliver the same therapeutic benefits as the natural herb, which contains at least another 60 cannabinoids in addition to THC. Recent research conducted by GW Pharmaceuticals in Great Britain has shown that Marinol is simply not as effective for pain management as the whole plant; a balance of cannabinoids, specifically CBC and CBD with THC, is what helps patients most. In fact, Marinol is not labeled for pain, only appetite stimulation and nausea control. But studies have found that many severely nauseated patients experience difficulty in getting and keeping a pill down, a problem avoided by use of inhaled cannabis.
Clinical research on Marinol vs. cannabis has been limited by federal restrictions, but a review of state clinical trials conducted in the 70’s and 80’s published in 2001 reports that “. . . the data reviewed here suggested that the inhalation of THC appears to be more effective than the oral route… Patients who smoked marijuana experienced 70-100% relief from nausea and vomiting, while those who used THC capsules experienced 76-88% relief.”[47] Additionally, patients frequently have difficulty getting the right dose with Marinol, while inhaled cannabis allows for easier titration and avoids the negative side effects many report with Marinol. As the U.K. House of Lords report states, “Some users of both find cannabis itself more effective.”
THE EXPERIENCE OF PATIENTS
Dorothy Gibbs
In 1911, at the age of one, I contracted the polio virus. . . The early onset of polio caused permanent damage in my legs, spine, and back, resulting in significant weakness and atrophy in my legs. As a result, I have never been able to walk without the assistance of crutches and braces or a wheelchair. Approximately 30 years ago, my condition began to deteriorate. I began to suffer from increasing levels of pain and weakness in my legs and back as well as severe osteoarthritis in my hands, arms, and joints. Over time, my deteriorating medical condition has been exacerbated by my pain, leaving me increasingly immobilized. . .
By May, 1996, my physician [Dr. Arnold Leff, M.D.] had tried various prescription medications to relieve my pain, including: Tylenol #3, Ultram, Daypro, Tegretol, Soma, Valium, steroid injections into the trigger point, Dilantin, Duragesic, Zofran and Comapazine for the nausea caused by the opioid pain relievers, and Doloboid and Lodine a nonsteroids. Nothing seemed to work, and the pain persisted. I was growing increasingly depressed by the inability of anything to relieve my pain. . .
During this period it was clear to me, my caretaker and my physician that nothing was working to combat my pain. My caretaker, Pat, had heard of the success some people experience with the medicinal use of marijuana for pain management. Sometime during the end of 1997, she obtained a sample for me. Although I had never used marijuana in my previous eighty-seven years of life, I was willing to try anything that could alleviate even part of the pain.
The relief I experienced from medical marijuana was almost immediate. I was so pleased with the result that I wrote to Dr. Leff about my use of medical marijuana and we talked about the benefits of the medicine. Dr. Leff examined me and noted that medical marijuana helped me experience less chronic pain and nausea, leading him to recommended medical marijuana as part of my daily pain care regimen….
Ever since trying medical marijuana, my life has drastically improved. Although chronic pain, related to my post-polio syndrome will always be a part of my life, medical marijuana had helped me manage this pain by providing fast and effective relief for my muscle spasms, acute pains, and arthritis. . . Since I began using medical marijuana, my pain is no longer persistent or debilitating. When I do suffer from pain, I am usually able to “get ahead of it” by using medical marijuana and make it manageable. . .
Margaret
I am a 45-year-old granny, and I smoke marijuana for medicinal reasons. I was 25 when I was diagnosed with rheumatoid arthritis. The doctor told me it was a painful, crippling disease and I would end up in a wheel chair. He gave me prescriptions for the arthritis and pain and sleeping pills.
Some of the pills had side effects and I would have to change to different ones. My arthritis was getting worse and I was depressed all the time. I started taking anti-depressants. For years I abused codeine, anti-depressants and sleeping pills. I don’t smoke tobacco or drink alcohol. My friends smoked marijuana but it didn’t interest me to try it.
I smoked my first joint when I was 30. One night I was in a lot of pain and feeling terribly uncomfortable. My friend Ed was with me and said he had heard marijuana helps relieve pain. I was willing to try anything and had a few tokes. After a few minutes I was relaxed and the pain seemed to have dulled. I was also more limber with my joints. I had a very restful sleep that night. I have been smoking marijuana every day since then. I have also been happier and no longer need anti-depressants. I now control my pain with marijuana.
Alfred
I’m a 23-year-old male currently employed as an accounting assistant. This fall I began work on my Master’s Degree. I am inflicted with Gout, a hereditary form of arthritis, which I have had for 6 years. When an attack arises the pain is in the main joint of my left foot and on the side of my big toe. When these attacks happen it is virtually impossible for me to walk.
I take Vicodin for the pain. I’m also given steroid shots for the pain in the doctor’s office. In addition, I take Allopurinol, this helps my body to get rid of the uric acid build up which leads to the pain of Gout. The reason that I have uric acid build up is because my kidneys do not function properly and rid my body of the uric acid.
The main side effects of Vicodin and Allopurinol are drowsiness, which are very bad if you are a full time college student and also employed. But, I have to have some kind of pain medicine to be able to walk, I have learned that marijuana helps a great deal with the pain, and I have found that I am able to walk and also function much better on marijuana than Vicodin. Allopurinol takes a terrible toll on my stomach. I would say 73% of the time I puke the medication up. I tried using marijuana in combination with the Allopurinol and I’ve found that this has helped drop the number of times that I throw the medication up. Now, I puke it up around 18% of the time, which is a big deal to me.
Matt Glandorf
I have arthritis in both hands and my chest, but here is the real kicker– I am severely allergic to aspirin. I can’t even take a Motrin without breaking out into a rash. I was born with a chest deformity called pectus excavatum (funnel chest and encaved chest are a couple other names for it.) I had corrective surgery in 1976 to try to make my rib cage bigger. In that surgery they break all the ribs and actually break the sternum in half, remove it, flip it over, and put it back together after removing most of the cartilage and muscle. Now I have arthritis along with lung problems and asthma. I usually spend two to three weeks a year in the hospital with lung infections and make numerous visits to the doctor for chest pain.
Needless to say, I have eaten a lot of pain killers and tried nerve blocks and so on. All have had little success and make me so stoned that I can’t even drive a car. So I started using pot and went from four Vicodin a day to one, and with watching my activities and a healthy diet I can go with no doctor’s meds for weeks on end.
Bob Burrill
I am a Canadian medical marijuana advocate. Osteoarthritis of the cervical spine is my problem. I have constant severe pain from many large bone spurs, compressed discs, and so on. Many narcotic and other types of pain reduction prescriptions have been tried with limited success. I have self-medicated with marihuana for the past 7 or 8 months, under my doctor’s care, with great success. My doctor and I have applied to the Canadian government to obtain a written ministerial exemption from prosecution so that I can cultivate and consume marijuana for a medical purpose.
Without medical marihuana, I have no life. I am restricted to bed or the couch and stuck inside the house. It’s about time governments and the public alike awakened to the fact that this is not “Cheech and Chong medicine” but one of the safest and user-friendly herbs on the planet. I only wish I had tried it a lot sooner. I can’t say enough about the merits and benefits of medical marijuana
The Experience of Doctors
Ethan Russo, M.D.
Patients have long told us that cannabis has been helpful to them in the treatment of their arthritic conditions. Science has now demonstrated that the THC component of cannabis is a very effective analgesic (pain killer), and that the CBD (cannabidiol) component has unique immunomodulatory benefits as an antagonist of tumor necrosis factor-alpha, supporting benefits in treatment of rheumatoid arthritis, as well as Crohn’s disease and psoriasis. It appears that cannabis-based medicines will likely be an important component of arthritis treatment in the 21st century.
Ethan Russo, MD, is a board-certified child and adult neurologist in Missoula, MT, and researcher in migraine, ethnobotany, medicinal plants, cannabis and cannabinoids in pain management. Dr. Russo currently serves in a consultancy position as Senior Medical Advisor to the Cannabinoid Research Institute, the division of GW Pharmaceuticals established to promote exploratory research. He holds faculty positions as adjunct associate professor in the Department of Pharmaceutical Sciences of the University of Montana, and clinical associate professor in the Department of Medicine of the University of Washington. He has published numerous articles in scientific journals and is co-editor of Cannabis and Cannabinoids: Pharmacology, Toxicology and Therapeutic Potential. Dr. Russo is the founding editor of Journal of Cannabis Therapeutics.
Arnold S. Leff, M.D.
I currently treat at least 20 patients for whom I believe marijuana is medically appropriate in responding to treatment-induced nausea or for appetite stimulation. In my medical judgment, in some cases medical marijuana may be the only effective medicine.
Two of my patients, Hal Margolin and Dorothy Gibbs, have benefited tremendously from [medical cannabis]. Both suffer from chronic pain. Ms. Gibbs, who is 93 years old and who had not previously tried marijuana until joining WAMM, has found marijuana to be a highly effective analgesic for treating acute and chronic pain associated with post-polio syndrome and complications arising there from [including arthritis].
Ms. Gibbs turned to marijuana only after trying a wide range of conventional prescription pharmaceuticals and therapies prescribed by me, but to little or no avail. These treatments, including powerful and highly addictive opioid analgesics, either did not work, gradually lost their efficacy, or caused such debilitating side effects (particularly nausea and dizziness) that Ms. Gibbs found intolerable.
Ms. Gibbs is a good example of a patient who experiences episodic acute pain for which Marinol is too slow-acting and who, when stricken with acute pain, often requires the faster analgesic and antiemetic effects produced by smoked marijuana. I have been pleasantly surprised at the degree to which marijuana has afforded Ms. Gibbs relief from the agony that she suffered.
Dr. Leff has been an advisor on national drug control policy and public health to the administrations of Presidents Nixon, Ford and Carter. He has worked with the Department of Defense and State Department developing drug abuse programs in foreign countries and for U.S. military troops, and has consulted with local law enforcement officials on drug treatment. He served as Director of Health Services for Contra Costa County, California and has held teaching positions on the medical school faculties of the University of Cincinnati and the University of California.
Harvey L. Rose, M.D.
Both my research and my many years as a clinician have convinced me that marijuana can serve at least two important roles in safe and effective pain management. Ample anecdotal evidence and clinical observations, as well as significant research findings, strongly indicate that marijuana, for whatever reason, is often effective in relieving pain. This is true across a range of patient populations, including the elderly, the terminally ill seeking comfort in their final days, young adults stricken with life-threatening conditions, and cancer patients unable to tolerate the devastating effects of potentially life-saving therapies. Marijuana is also widely recognized as an antiemetic that reduces the nausea and vomiting often induced by powerful opioid analgesics prescribed for chronic, severe pain, as well as the nausea, vomiting and dizziness which often accompany severe and/or prolonged pain. I have had the benefit of consultations on this subject over many years with a range of treatment providers, including physicians, oncologists, pharmacologists, family practitioners, hospice workers, and pain specialists. . .
Specifically, I have found that cannabis can have an important opioid-sparing effect for pain patients. That is to say, that patients who are prescribed high doses of opioid analgesics can significantly reduce their reliance on these medications and improve their daily functioning by incorporating cannabis into their pain care regimen.
Marijuana not only has important analgesic properties but it also is an effective and important adjuvant therapy for patients suffering acute and/or chronic pain. No experienced and respected physician will deny that for such patients opioid therapy is central to palliative care. By the same token, the same experienced physicians will readily acknowledge that opioids often induce nausea and vomiting. For a number of pain patients, standard prescription antiemetics (e.g., Compazine, Zofran and Reglan) simply do not substantially reduce their nausea. For many, those medications are substantially less effective, or produce more debilitating side effects, than marijuana. . .
Quite simply, marijuana can serve much the same function for pain patients undergoing opiate therapy that it does for cancer patients undergoing chemotherapy: it suppresses the nausea and vomiting associated with treatment, and reduces the pain associated with prolonged nausea and retching, thereby increasing the chances that the patient will remain compliant with the primary treatment. With both chemotherapy and long-term pain management, failure to obtain and continue proper palliative and adjutant care can have dire, even fatal, consequences. . .
Finally, it is important to note that in my clinical experience observing patients who ingest cannabis for relief from pain and nausea and/or to stimulate appetite, I have witnessed no adverse complications. By contrast, many of the first-line pharmaceuticals used to combat cancer, HIV/AIDS, and pain associated with these and other illnesses can induce a variety of iatrogenic effects, including, in some instances, death. While patients may face serious legal implications related to their use of medical marijuana, as a physician I have yet to encounter a medical downside to their cannabinoid therapy. . .
[A]gainst the backdrop of a growing body of scientific research, the reports of myriad pain patients, and the burgeoning clinical experience of physicians like myself, it is my considered opinion that cannabis can constitute an acceptable and sometimes necessary medicine to alleviate the immediate suffering of certain patients. Dr. Rose served as a medical officer in the Air Force before entering private practice. During his 40-year career, he has taught at UC Davis School of Medicine and consulted with state legislative bodies.
Research Citations
11. Abrams, Donald I., et al (2003). Short-Term Effects of Cannabinoids in Patients with HIV-1 Infection: A Randomized, Placebo-Controlled Clinical Trial. Ann Intern Med. 2003 Aug 19;139(4):258-66.
12. Bab I and Zimmer A (2008). Cannabinoid receptors and the regulation of bone mass. Br J Pharmacol 153(2):182-188.
13. Buckley NE, et al (2000) .Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol 396(2-3):141-149.
14. Idris AI, et al (2009). Cannabinoid receptor type 1 protects against age-related osteoporosis by regulating osteoblast and adipocyte differentiation in marrow stromal cells. Cell Metab 10(2):139-147.
15. Ofek O, et al (2006). Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci U S A 103(3):696-701.
16.Tam J, et al (2006). Involvement of neuronal cannabinoid receptor CB1 in regulation of bone mass and bone remodeling. Mol Pharmacol 70(3):786-792.
17.Tam J, et al (2008). The cannabinoid CB1 receptor regulates bone formation by modulating adrenergic signaling. Faseb J 22(1):285-294.
18. Huang QY, et al (2009). Multiple osteoporosis susceptibility genes on chromosome 1p36 in Chinese. Bone 44(5):984-988.
19. Karsak M, et al (2005). Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum Mol Genet 14(22):3389-3396.
20.Karsak M, et al (2009). The cannabinoid receptor type 2 (CNR2) gene is associated with hand bone strength phenotypes in an ethnically homogeneous family sample. Hum Genet.
21. Russo EB (2002). Role of cannabis and cannabinoids in pain management. In: Weiner RS, editor. Pain management: A practical guide for clinicians. 6th ed. Boca Raton, FL: CRC Press. p. 357-375.
22. Marcandier M (1764). Treatise on hemp. London: T. Becket and P.A. de Hondt.
23. Formukong E et al (1988). Analgesic and Antiinflammatory Activity of Constituents of Cannabis Sativa L. Inflammation 12: 361.
24. Barret ML et al (1985). Isolation from Cannabis sativa L. of Cannflavon – a novel inhibitor of prostaglandin production. Biochem. Pharmacol. 34: 2019
25. Burstein SH et al (1989). Antagonism to the actions of platelet activating factor by a nonpsychoactive cannabinoid. J Pharmacol. Exp. Therap. 251: 531-5
26. Sofia RD (1989). Antiedemic and analgesic properties of delta-9-THC compared with three other drugs. Eur. J. Pharamacol. 41: 705-9
27. Zurier RB et al (1998). Dimethylheptyl-THC-11 Oic Acid: A Nonpsychoactive Antiinflammatory Agent with a Cannabinoid Template Structure. ARTHRITIS AND RHEUMATISM January; volume 41, number 1, pages 163-170.
28. Costa B et al (2004). Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedebergs Arch Pharmacol. Mar;369(3):294-9. Epub 2004 Feb 12.
29. Malfait AM et al (2000) .The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. Aug 15 97(17):9561-6.
30. James JS (1998). Marijuana, inflammation, and CT-3 (DMH-11C): cannabis leads to new class of antiinflammatory drugs. AIDS Treat News. Jan 23;(No 287):1, 5.
31. Straus SE (2000). Immunoactive cannabinoids: Therapeutic prospects for marijuana constituents. Proc Natl Acad Sci U S A. Aug 15 97(17):9563.
32. Shohami E (2001). Nature. Oct 4;413(6855):527-31.
33. Burstein SH (2000). Ajulemic acid (CT3): a potent analog of the acid metabolites of THC. Curr Pharm Des. Sep 6(13):1339-45.
34. Burstein SH et al (2004). Ajulemic acid: A novel cannabinoid produces analgesia without a “high”. Life Sci. Aug 6;75(12):1513-22.
35. Devane WAet al1(1992). Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science.258:1946-1949.
36. Barg J et al (1995). Cannabinomimetic behavioral effects of andadenylate cyclase inhibition by two new endogenous anandamides. Eur J Pharmacol.;287:145-152.
37. Klein TW et al (1998). Cannabinoid receptors and immunity. Immunol Today. 797:225-233.
38. Daaka Y et al (1996). Cannabinoid receptor proteins are increased in jurkat, human T-cell line after mitogen activation. J Pharmacol Exp Ther. 276:776-783.
39. Kaminski NE (1996); Immune regulation by cannabinoid compounds through the inhibition of the cyclic AMP signaling cascade and altered gene expression. Biochem Pharmacol; 52(8):1133-40.
40. Di Marzo V (1998). ‘Endocannabinoids’ and other fatty acid derivatives with cannabimimetic properties: biochemistry and possible physiopathological relevance. Biochimica et Biophysica Acta.1392(2-3):153-75.
41. Smith PB et al (1994). The pharmacological activity of anandamide, a putative endogenous cannabinoid in mice. J Pharmacol Exp Ther. 270:219-227.
42. Burstein SH (2000). Ajulemic acid (CT3): a potent analog of the acid metabolites of THC. Curr Pharm Des. Sep;6(13):1339-45.
43. Zurier RB et al (2003). Suppression of human monocyte interleukin-1beta production by ajulemic acid, a nonpsychoactive cannabinoid. Biochem Pharmacol. Feb 15;65(4):649-55.
44. Hazekamp A et al (2006). Evaluation of a vaporizing device (Volcano(R)) for the pulmonary administration of tetrahydrocannabinol. J Pharm Sci 95 (6) Apr 24: 1308-1317.
45. Sidney S et al (1997). Marijuana Use and Cancer Incidence. Cancer Causes and Control; 8: 722-728.
46. Tashkin D (2006). Marijuana Use and Lung Cancer: Results of a Case-Control Study. American Thoracic Society International Conference. May 23, 2006.
47. Musty R, Rossi R (2001). Effects of smoked cannabis and oral delta-9-tetrahydrocannabinol on nausea and emesis after cancer chemotherapy: a review of state clinical trials. Journal of Cannabis Therapeutics. 1: 29-56.
Read the full article at the source: Safe Access Now



No Comment